Mechanical Engineering
Fiery explosions of hot metal on water
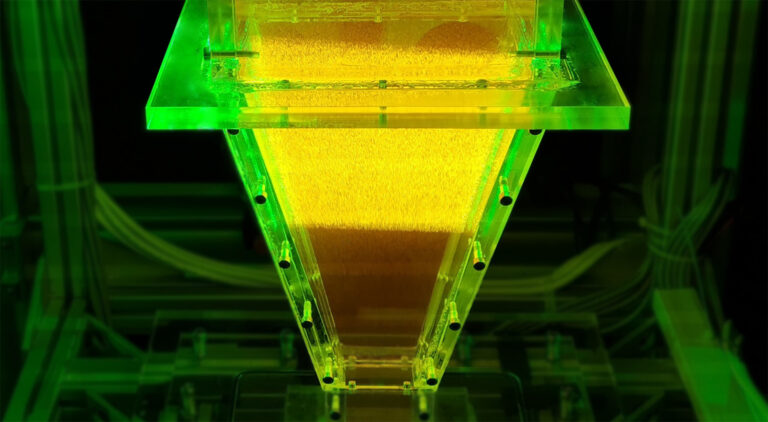
Images of from a high-speed camera reveal a microbead formation process during the vapor explosion of liquid metal dropping into a pool of water.

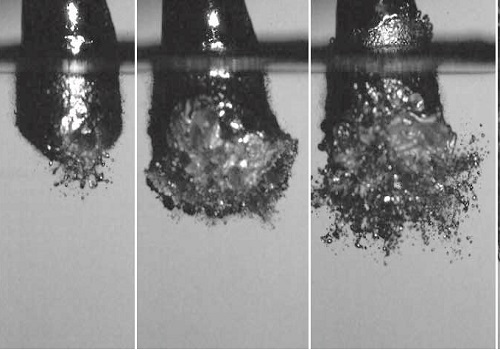
Vapor explosion of an 8 mm diameter drop of liquid Field’s metal as it falls into a pool of water.
© 2016 KAUST
The explosive reaction of a liquid metal dropping into water has been captured with high-speed cameras by researchers from KAUST. The images reveal how the explosive formation of water vapor around the liquid metal influences the shape of the metal as it hardens1. For certain metals, perfectly-shaped microbeads are created during the powerful reaction with water.
Immersion of hot liquids in other liquids is not uncommon, even outside of the laboratory. An example is hot lava from a volcanic eruption encountering water reservoirs or flowing into the sea. This interaction can lead to dramatic reactions when vapor layer forms around the liquid metal. The vapor layer can become unstable and quickly expands into hot clouds of water and ash, noted Siggi Thoroddsen from the KAUST High-Speed Fluids Imaging Laboratory, who also led the research team.
“This happened when the Icelandic volcano Eyjafjallajökull erupted in 2010 and grounded airplanes all over Europe,” he said.
The researchers studied related reactions in the lab using a metal alloy known as Field’s metal, which melts at low temperatures of around 60 degrees Celsius. With experiments conducted at 550 degrees Celsius metal temperature, the transfer of energy between the metal and the water is very violent. KAUST Ph.D. student Nadia Kouraytem wore a full protective facial mask and a fire-resistant lab coat during these experiments. High-speed cameras captured the explosive process at speeds of up to 50,000 frames per second.
The images obtained were dramatic and showed an explosive reaction that tore the metal apart. In the case of Field’s metal, small spherical microbeads formed during the process.
During the reaction, the metal transitioned through several stages with increasing ferocity. While initially only a small part of the metal interacted with the water, over a longer period increasingly more of the metal was exposed and took part in the reaction until the disintegration of the liquid metal into small beads.
The unusual microbead formation occurs due to the low melting temperature. Metals with a higher melting temperature (such as tin) solidify faster because their higher solidification temperature is reached more quickly upon cooling so that there is less time for the material to disintegrate. An example is the porous structures seen in solidified lava from volcanic eruptions.
In the case of Field’s metal, the beads are highly uniform, and it will be interesting to study their creation processes further, noted Thoroddsen.
“In future experiments, we want to better control the original drop, change its size and impact velocity. This should further probe the instabilities of the vapor layer that forms around the metal,” he said.
References
-
Kouraytem, N., Li, E. Q. & Thoroddsen, S.T. Formation of microbeads during vapor explosions of Field’s metal in water. Physical Review E 93, 063108 (2016).| article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Mechanical Engineering
Falling water forms beautiful fluted films
Mechanical Engineering
Innovative strain sensor design enables extreme sensitivity
Mechanical Engineering
Turbulent flow shows surprise patterns that could help boost efficiency

Mechanical Engineering



