Material Science and Engineering
A golden replacement
Altering a single atom in a silver nanocluster considerably changes its properties, creating an exciting opportunity to design these clusters.
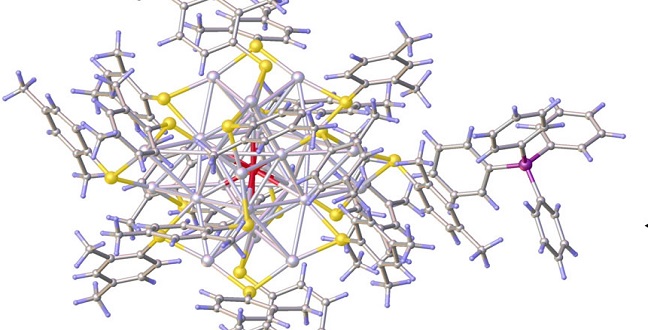
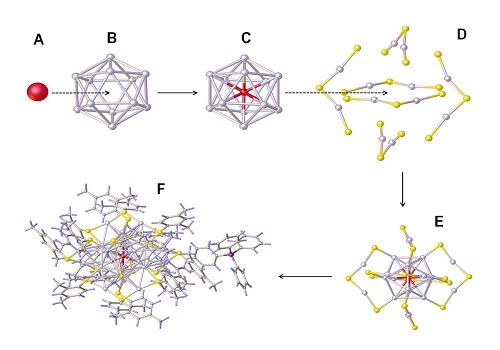
Composing a silver nanocrystal: the center silver atom (a) surrounded by a cage of 12 other silver atoms (b) embedded by further atoms (c) and stabilized by further ligands (d).
Reproduced with permission from ref erence1.© 2016 John Wiley and Sons.
The appearance of metals, such as their shiny surface or their electrical conductivity, is determined by the ensemble of atoms that comprise the metal. The situation differs on the molecular scale, and KAUST researchers have shown that replacing a single atom in a cluster of 25 silver atoms with one gold atom fundamentally changes its properties1.
Metal atom nanoclusters are made from a core of a few metal atoms surrounded by a protective shell of stabilizing ligands. Nanoclusters come in different sizes, but each stable variation of nanoclusters has exactly the same number of metal atoms. This leads to very controllable properties, noted Osman Bakr, KAUST associate professor of material science and engineering and leader of the research team.
“Nanoclusters have unique arrangements of atoms and size-dependent absorption, fluorescence, electronic and catalytic properties,” he said.
A popular metal nanocluster is [Ag25(SR)18]–, which consists of of 25 silver atoms. This nanocluster is unique as it corresponds to a gold nanocluster that has exactly the same number of atoms. Both clusters have different properties due to the different metal used. To understand how exactly the atomic composition affects these properties, the researchers replaced a single silver atom with gold.
Replacing a single atom in a nanocluster is a difficult task. Direct chemical methods can be used, but these give little control over how many atoms are replaced, making it difficult to ascribe particular properties to the nanocluster structure.
Instead, the researchers used a galvanic replacement process that relies on difference in the electrochemical potential between the incoming and outgoing atoms to induce atomic replacements. To their surprise, the process produced a reliable and precise atomic exchange in which only the center silver atom is replaced by gold.
The replacement yielded dramatic changes in the nanocluster. A solution of the silver nanoclusters appears orange, whereas after the replacement of the central atom the color turns dark green.
This indicates more fundamental changes in properties, Bakr said. “The ambient stability and fluorescence of the nanocluster were enhanced by a factor of 25 as a result of this single atom replacement. Furthermore, we are now able to demonstrate the importance of a single atom impurity on nanoparticles and modulate the properties at the single atom level,” he noted.
The reliable replacement of only a single gold atom opens the door to a more systematic investigation of metal nanoclusters, which can help to uncover the mechanisms of the chemical and physical changes arising from the replacement.
References
-
Bootharaju, M.S., Joshi, C.P., Parida, M.R., Mohammed, O.F. & Bakr, O.M. Templated atom-precise galvanic synthesis and structure elucidation of a [Ag24Au(SR)18]– nanocluster. Angewandte Chemie International Edition 55, 922 –926 (2016).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics



