Bioscience
A deadly delivery for breast cancer
Zinc oxide (ZnO) nanoparticles may help destroy difficult-to-treat triple-negative breast cancer tumors.
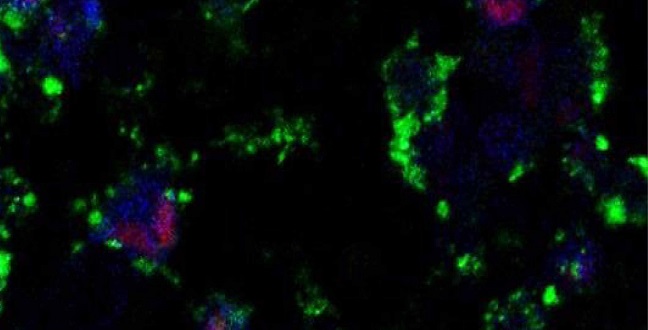
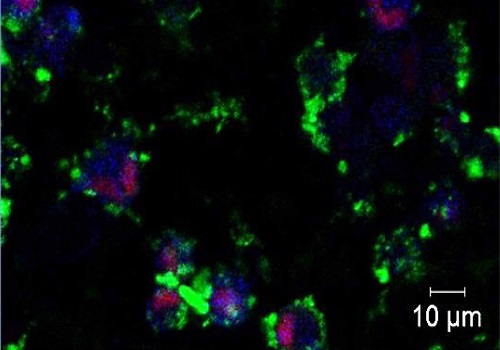
Live cell imaging shows fluorescently labeled targeted ZnO nanoparticles (green) dissolving to release zinc ions (blue) within the intracellular environment of triple-negative breast cancer cells, activating cell death pathways (red).
© 2016 KAUST
Breast tumors that are so-called triple-negative are largely resistant to existing cancer drugs, giving patients an especially poor prognosis. KAUST researchers and collaborators are now exploring a potential therapeutic option for such tumors that is based on the tumor-specific effects of zinc oxide (ZnO) nanoparticles1.
Previous studies have shown that ZnO treatment can kill cancerous cells while leaving healthy cells intact. However, the mechanism is poorly defined; some data suggest that ZnO particles are ingested by and dissolve within tumor cells, while other findings suggest that cells absorb free zinc released when these particles dissolve in the acidic tumor environment.
In a study led by Jasmeen Merzaban, KAUST assistant professor of bioscience, and Alexandra Porter from Imperial College London, Ph.D. students Basma Othman and Ayman Abuelela used microscopy to observe what happened when triple-negative breast tumor cells were treated with ZnO. For comparison, they used both untargeted conventional ZnO nanoparticles as well as targeted nanoparticles tagged with a peptide that specifically recognizes a protein found on the surface of cancer cells.
The results unambiguously supported a model in which the nanoparticles are consumed by the cells before inducing cell death.
“We were able to observe in real time the uptake of both classes of ZnO nanoparticles and confirm the subsequent rise in zinc ion concentrations within cells prior to their death,” said Merzaban.
The targeted nanoparticles proved substantially more effective at killing triple-negative cells in culture, requiring lower doses of ZnO. However, the real-time imaging experiments also revealed considerable variability in the way individual cells respond to treatment, with some cells perishing quickly while others hung on.
The researchers speculate that this heterogeneity might reflect the presence of relatively drug-resistant cancer stem cell subpopulations.
“These cells are believed by many to be responsible for the relapse of cancer following traditional therapies such as chemotherapy,” noted Merzaban.
Eliminating such cancer stem cells could be a major victory against hard-to-treat tumors. The researchers are now exploring whether such cells are responsible for the heterogeneous response observed in their experiments, with an eye toward improving the efficacy of their approach.
In parallel, Merzaban plans to initiate animal studies that might give better insights into the clinical applicability of ZnO.
“We intend to use in vivo imaging tools to determine how effective these nanoparticles are at inducing the death of breast cancer cells in tumor mouse models,” she said. “We are very excited about the potential of these and other nanoparticles as part of the future of medicine.”
References
-
Othman, B.A., Greenwood, C., Abuelela, A.F., Bharath, A.A., Chen, S. et al. Correlative light-electron microscopy shows RGD-targeted ZnO nanoparticles dissolve in the intracellular environment of triple negative breast cancer cells and cause apoptosis with intratumor heterogeneity. Advanced Healthcare Materials 5, 1310-1325 (2019).| article
You might also like

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience
AI speeds up human embryo model research

Bioscience



