Mechanical Engineering
Shaping rate rules to fuel the future
Precise rate constants could provide high-fidelity combustion models for cleaner and more efficient fuels.
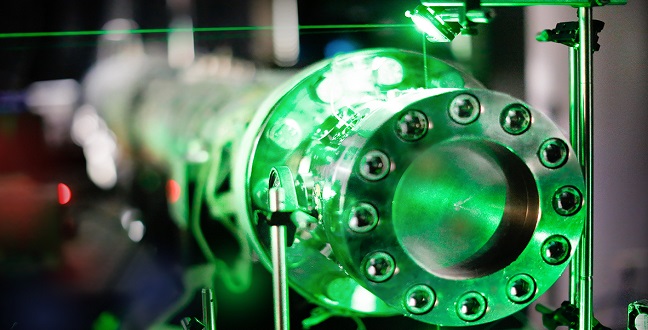
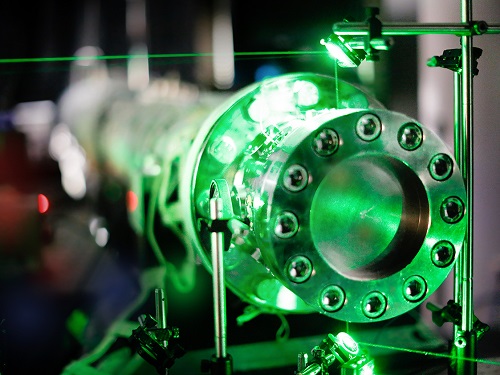
End section of the shock tube. A green laser beam passes through the optical ports of the shock tube.
© 2016 KAUST
The performance of future engines could soon be predicted with greater accuracy thanks to research by KAUST scientists to find high-efficiency and low-emission solutions in the fight against climate change.
Engine design and optimization depend on combustion chemistry models consisting of multiple elementary reactions. In particular, reactions between hydrocarbons and hydroxyl (OH) radicals play a central role in the breakup of fuel components during combustion.
Few researchers, however, have directly measured rate constants for high-temperature reactions between OH and large alkanes comprising more than five carbon atoms; these prevail in gasoline and diesel.
To shed light on this, Aamir Farooq and colleagues from the KAUST Clean Combustion Research Center have established ‘rate rules’, or site-specific rate constants, for the OH-mediated hydrogen removal from alkanes at carbons bearing two and three functional groups1.
In this approach, they evaluated the rate constants for the high-temperature reactions between large linear and branched alkanes and OH radicals.
“We carefully selected seven alkanes varying in structure to derive rate rules applicable to alkanes not covered in this study,” Farooq noted.
The researchers conducted their measurements in a shock tube, an ideal reactor for high-temperature kinetic studies. A supersonic wave traveled through the test gas mixture, heating it to temperatures ranging between 880 and 1440 K almost instantly. Farooq explained that a large pressure discontinuity is created between the test gas mixture and an inert gas (such as helium) using a diaphragm. The diaphragm bursts when the inert gas pressure increases, producing the supersonic wave.
Test gas mixtures combined a specific alkane, a clean thermal OH radical source called tert-butyl-hydroperoxide, and argon as a thinner. At high temperature, tert-butyl-hydroperoxide decomposed in a few microseconds to generate the radicals, which then reacted with the alkane. The researchers determined the rate constants by monitoring the OH radical decay using an ultraviolet laser system.
Measured rate constants for n-hexane and 2,2-dimethyl-butane closely matched previous assessments, supporting the researchers’ approach. The value calculated using the rate rules also agreed with experimental data for the reaction involving 2-methyl-heptane.
“This gives us confidence that our rate rules are suitable for other classes of alkanes,” said Farooq. These measurements were also in line with estimates obtained by earlier prediction methods.
The rate constants and rate rules are now implemented in new combustion models developed at KAUST and at other international universities.
“These models are used by our collaborators from Saudi Aramco and other institutes in high-fidelity engine simulations to improve engine efficiency and reduce emissions,” Farooq added.
References
- Badra, J., Elwardany, A. & Farooq, A. Shock tube measurements of the rate constants for seven large alkanes + OH, Proceedings of the Combustion Institute 35, 189–196 (2015).I article
You might also like

Mechanical Engineering
Cracking clean fuel combustion

Mechanical Engineering
Tiny sensor could transform head injury detection
Mechanical Engineering
Electrocatalytic CO2 upcycling excels under pressure
Chemical Engineering
Rethinking machine learning for frontier science

Mechanical Engineering
Falling water forms beautiful fluted films
Mechanical Engineering
Innovative strain sensor design enables extreme sensitivity
Mechanical Engineering
Turbulent flow shows surprise patterns that could help boost efficiency

Mechanical Engineering



