Material Science and Engineering
Clear detection of ultraviolet light
Transparent crystals that grow from heated solutions offer an improved material for detecting ultraviolet light.
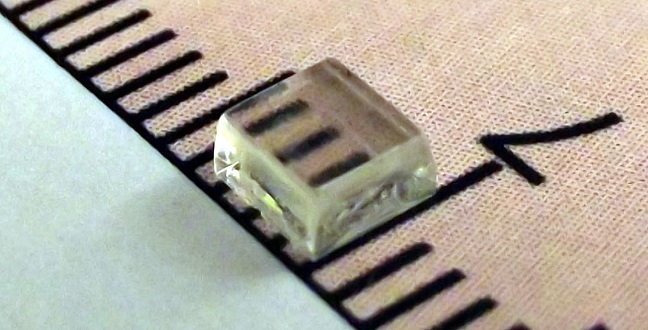
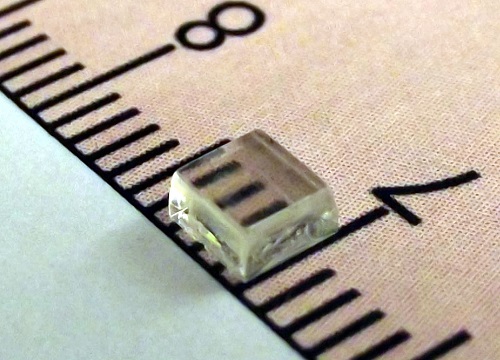
The novel UV-photodetecting single crystalsare transparent to visible light.
© 2015 KAUST
Single semiconducting crystals grown by KAUST researchers are showing strong potential as candidates for the next generation of opto-electronic devices whose electrical properties respond to light. The crystals can be grown up to several millimeters wide and have the advantages of being transparent to both visible and infrared light as well as sharply sensitive in the ultraviolet (UV) range1.
Their most obvious application is in UV-photodetectors. These are used widely in systems such as chemical pollution sensors, flame sensors in fire alarms and secure communications systems. The crystals will suit most situations that require the detection of UV light by producing an electrical signal, especially where ignoring other wavelengths of light is important.
“Most existing UV-photodetectors suffer from limitations such as the need for filters to block visible and infrared light and difficult production methods,” explained Osman Bakr from the Solar & Photovoltaics Engineering Research Center (SPERC) at KAUST.
The crystals manufactured by Bakr and colleagues are naturally sensitive to only ultraviolet light and are easy and inexpensive to prepare from chemical solutions. The UV light provides the energy to stimulate the conductive state, creating an electrical signal. Bakr reported that the semiconducting properties of his single crystals are comparable to those of the best quality silicon, the traditional basis of most semiconductors.
The crystals are made of materials known as “hybrid perovskites.” These have a similar crystal geometry to natural perovskite minerals, but they have alternative chemical groups in place of the calcium, titanium and oxygen atoms of the natural materials. The crystals produced at KAUST contain carbon, hydrogen, nitrogen, lead and chlorine. The presence of the chlorine in the form of chloride ions (Cl–) proved crucial to obtain the desired characteristics.
Most types of crystals form when solutions of materials in solvents are cooled, but the formation of these hybrid perovskites came through an unusual reversal of that phenomenon.
“While studying some similar materials, we noticed that their crystals formed at high temperatures rather than on cooling,” Bakr explained.
This observation eventually revealed that the best chloride-based UV-photodetecting crystals also required high temperatures for the crystals to form. This “inverse temperature crystallization” process was refined through careful selection of solvents to yield very high-quality single crystals.
The KAUST team is also exploring the possibilities for preparing crystals that detect other wavelengths of light by varying their chemical composition.
“We have already made bromide-based perovskite microcrystals for fast and ultrasensitive visible light photodetector applications,” Bakr said.
References
- Maculan, G., Sheikh, A. D., Abdelhady, A. L., Saidaminov, M. I., Haque, M. A. et al. CH3NH3PbCl3 single crystals: inverse temperature crystallization and visible-blind UV-photodetector. The Journal of Physical Chemistry Letters 6, 3781–3786 (2015).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel

Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike

Applied Physics
Two-dimensional altermagnets could power waste heat recovery

Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan

Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics



