Material Science and Engineering
Tip tricks control reactions in a single molecule
Pulses from an atom-sharp tip enable researchers to break and form chemical bonds at will.
Chemical reactions often produce messy mixtures of different products. Hence, chemists spend a lot of time coaxing their reactions to be more selective to make particular target molecules. Now, an international team of researchers has achieved that kind of selectivity by delivering voltage pulses to a single molecule through an incredibly sharp tip.
“Controlling the pathway of a chemical reaction, depending on the voltage pulses used, is unprecedented and very alluring to chemists,” says KAUST’s Shadi Fatayer.
The team used an instrument that combines scanning tunneling microscopy (STM) and atomic force microscopy (AFM). Both techniques can map out the positions of atoms within individual molecules using a tip that may be just a few atoms wide. But the voltage can also be used to break bonds within a molecule, potentially allowing new bonds to form.
“Tip-controlled reactions have been previously performed, but there was no control over the final product,” Fatayer says. “The selectivity is the key element here — depending on the polarity and value of the voltage pulses, we can form and break different internal bonds at will.”
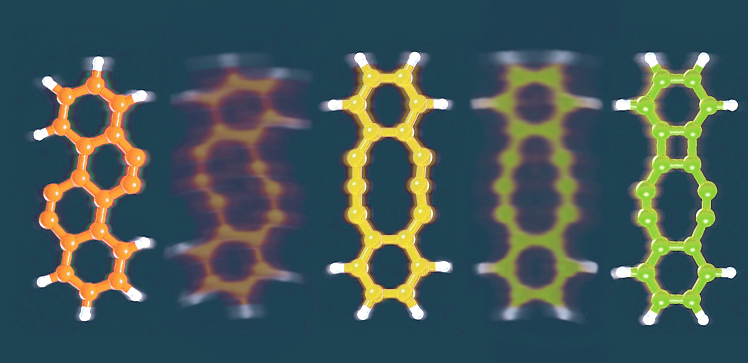
The researchers used this approach to study tetrachlorotetracene, a molecule that contains four chlorine atoms attached to a row of four hexagonal rings of carbon atoms. Applying a voltage of about 3.5V removed two chlorine atoms and prompted the molecule to rearrange. Increasing the voltage removed the remaining chlorine atoms, triggering further rearrangements that formed three different products.
The first product has four hexagonal rings arranged in a zig-zag pattern; the second has a 10-membered ring flanked by two six-membered rings; and the third contains a four-membered ring, an eight-membered ring and two six-membered rings.
Small voltage pulses could be used to interconvert these products. By fine-tuning the voltage, the researchers could control which bonds were broken and which rearrangement product formed.
Combining their results with theoretical calculations, the researchers showed that the method’s selectivity depends on the landscape of energy states the molecules adopt when they carry different electrical charges, known as their oxidation state. Since the initial oxidation state of a molecule can be controlled by an electric field, this approach could help chemists to design new chemical reactions and products, Fatayer says. This research was featured on the front cover of Science.
His group is now developing ways to add to or remove single electrons from individual molecules and to apply voltage pulses to specific parts of a molecule to control which chemical reaction occurs.
References
-
Albrecht, F., Fatayer, S., Pozo, I., Tavernelli, I., Repp, J., Peña, D. & Gross, L. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 337, 298-301 (2022).| article
You might also like

Chemistry
Turning infrared solar photons into hydrogen fuel
Applied Physics
A single additive enables long-life, high-voltage sodium batteries

Bioengineering
Smart patch detects allergies before symptoms strike
Applied Physics
Two-dimensional altermagnets could power waste heat recovery
Applied Physics
Interface engineering unlocks efficient, stable solar cells

Applied Physics
The right salt supercharges battery lifespan
Applied Physics
Light-powered ‘smart vision’ memories take a leap forward

Applied Physics




