Environmental Science and Engineering
Strong sunlight powers passive cooling device
Sustainable electricity-free appliance can harness solar energy to reduce temperatures on hot days.
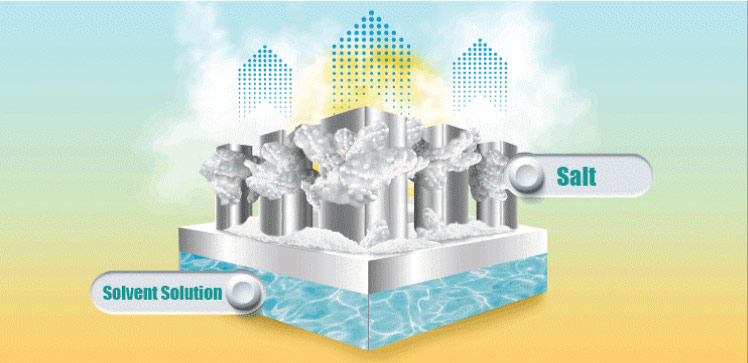
A simple cooling system driven by the capture of passive solar energy could provide low-cost food refrigeration and living space cooling for impoverished communities with no access to the electricity grid. The system, which has no electrical components, exploits the powerful cooling effect that occurs when certain salts are dissolved in water. After each cooling cycle, the system uses solar energy to evaporate the water and regenerate the salt, ready for reuse.
“Hot regions have high levels of solar energy, so it would be very attractive to use that solar energy for cooling,” says Wenbin Wang, a postdoc in Peng Wang’s lab. In many parts of the world, there is a greater need for cooling because of climate change, but not every community can access electricity for air conditioning and refrigeration. “We conceptualized an off-grid solar-energy conversion and storage design for green and inexpensive cooling,” Professor Wang says.

The cooling system designed by KAUST engineers could be used to cool rooms in households.
© 2021 KAUST; Wenbin Wang
The team designed a two-step cooling and regeneration system, with the cooling step based upon the fact that dissolving certain common salts in water absorbs energy, which rapidly cools the water. After comparing a range of salts, ammonium nitrate (NH4NO3) proved to be the standout performer, with a cooling power more than four times greater than its closest competitor, ammonium chloride (NH4Cl). The ammonium nitrate salt’s exceptional cooling power can be attributed to its high solubility. “NH4NO3’s solubility reached 208 grams per 100 grams of water, whereas the other salts were generally below 100 grams,” Wenbin says. “This salt’s other advantage is that it is very cheap and already widely used as fertilizer,” he adds.
The system has good potential for food storage applications, the team showed. When the salt was gradually dissolved in water in a metal cup placed inside a polystyrene foam box, the temperature of the cup fell from room temperature to around 3.6 degrees Celsius and remained below 15 degrees Celsius for over 15 hours.
Once the salt solution reached room temperature the team used solar energy to evaporate the water using a bespoke cup-shaped 3D solar regenerator. The cup was made from a material designed to absorb as much of the solar spectrum as possible. As the water evaporated, the NH4NO3 crystals grew over the cup’s outer wall. “The crystallized salt can be collected automatically as the salt drops off due to gravity,” Wenbin says.
Once collected, the salt effectively represents a stored form of solar energy, ready to be reused for cooling again when required.
References
- Wang, W., Shi, Y., Zhang, C, Li, R., Wu, M., Zhuo, S., Aleid, S. & Wang, P. Conversion and storage of solar energy for cooling. Energy and Environmental Science 15, 136-145 (2022).| article
You might also like

Environmental Science and Engineering
Ultrathin water repellent membrane advances desalination
Environmental Science and Engineering
Bacteria reveal hidden powers of electricity transfer

Environmental Science and Engineering
Wastewater surveillance tracks spread of antibiotic resistance

Bioscience
Super fungi survive extreme Mars-like environments

Environmental Science and Engineering
Rethinking food systems to restore degraded lands

Environmental Science and Engineering
Combat climate change by eliminating easy targets

Environmental Science and Engineering
Wastewater treatment to fight the spread of antibiotic resistance
Bioscience




