Bioscience
Elegant constrictions in a cellular kill switch
Combining expertise has shed new light on how cells self-destruct during microbial infection.
After eating the E. Coli bacteria (in red), the normal macrophages (shown on the left) die in a process called pyroptosis, whereas the PANX1-deficient macrophages fail to initiate pyroptosis and survive (right). © 2021 KAUST
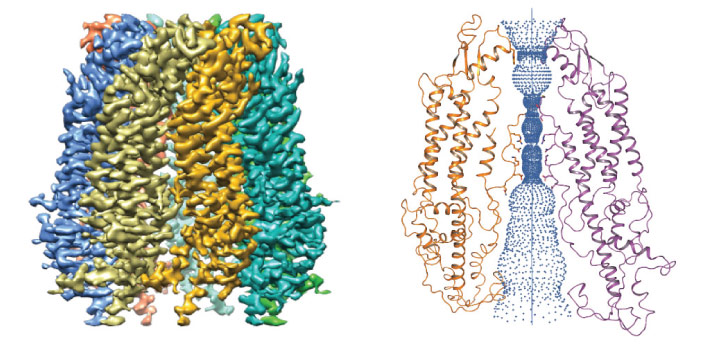
The inner workings of a “self-destruct switch” present on human cells that can be activated during an immune response have been revealed. In unprecedented detail, KAUST scientists with collaborators in China report the 3D atomic structure of the human PANX1 protein, which may help underpin new therapies that target the immune system.
When cells become infected with a pathogen, the body’s immune system works to destroy the infected cells before they become a threat to surrounding tissues. This form of cell death, during which a cell releases potent danger signals to recruit immune cells, is known as pyroptosis.

Ph.D. student Baolei Yuan (left) and Prof. Mo Li (right) study the super-resolution images showing the cellular localization of the PANX1 protein.
© 2021 KAUST; Anastasia Serin
The protein PANX1, a channel pore that dots a cell’s outer membrane, has been implicated in pyroptosis because it allows the passage of ions and molecules out of the cell, which helps mark it for destruction. But how it carries out this function, or “flicks the switch” on cell death, has been unclear.
After eating the E. Coli bacteria (in red), the normal macrophages (shown on the left) die in a process called pyroptosis, whereas the PANX1-deficient macrophages fail to initiate pyroptosis and survive (right). © 2021 KAUST
“We wanted to know the gating mechanisms of PANX1 by resolving the previously unrevealed protein ends — the C- and N-termini — to understand their importance in pyroptosis,” says study co-first author Baolei Yuan, a Ph.D. student in Mo Li’s lab.
Li’s collaborators, led by Maojun Yang at Tsinghua University, first isolated the protein and revealed the 3D structure using data collected on KAUST’s state-of-the art Titan Krios cryo-transmission electron microscope and a similar instrument at Tsinghua University.

The team revealed the protein’s 3D structure using KAUST’s state-of-the-art Titan Krios cryo-transmission electron microscope.
© 2021 KAUST; Anastasia Serin
Through this, the researchers visualized a number of amino acids within the protein that “pinch” the pore to control the passage of molecules across the cell membrane. Using cultured cells, Li’s team confirmed the indispensable role these amino acids and PANX1 play in pyroptosis.
But the molecular details of how ions and molecules cross the PANX1 pore only became clear when the researchers teamed up with Xin Gao, whose group was able to simulate the molecular dynamics.
“I was surprised by the intricate and beautifully arranged constrictions in the permeation path of the PANX1 channel,” says Yuan.

The unprecedented level of insight of the PANX1 protein provides a high-quality reference for potential drug targets.
© 2021 KAUST; Anastasia Serin
Together, the cryo-EM and molecular dynamics data revealed that the N- and C-termini stretch deeply into the pore to form barriers under normal conditions to keep ions and small components inside the cells. But once stimulated, the two termini are either modified or cleaved to make the channel more permeable, releasing molecules that help destroy the cell.
“These findings give us a much better understanding of the mechanism that controls pyroptosis,” says Li. “PANX1 has been associated with diverse and numerous pathophysiological conditions related to the immune system. Our study provides a high-quality reference for potential drug targets.”
References
-
Zhang, S., Yuan, B., Lam, J.H., Zhou, J., Zhou, X., Mandujano, G.-R., Tian, X., Li, Y., Han, R., Li, M. & Yang, M. Structure of the full-length human Pannexin1 channel and insights into its role in pyroptosis. Cell Discovery 7, 30 (2021).| article
You might also like

Bioengineering
Bio-inspired network structures for next-generation AI

Bioscience
Robust workflow built for chemical genomic screening

Bioscience
Cell atlas offers clues to how childhood leukemia takes hold

Bioscience
Hidden flexibility in plant communication revealed

Bioscience
Harnessing the unintended epigenetic side effects of genome editing

Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities

Bioscience




