Bioscience
Biobank of stem cells helps understand genetic abnormalities
A database of newly generated stem cell lines could provide the key to understanding the genetic factors that drive Klinefelter syndrome and related diseases.
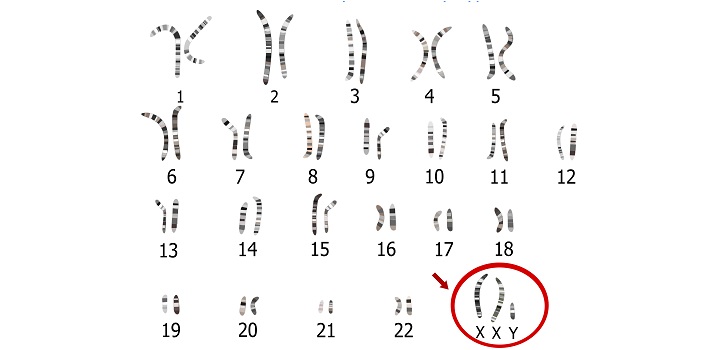
The largest cohort of stem cell lines derived from patients with Klinefelter syndrome has been developed by KAUST researchers. These powerful cellular tools could be used to develop regenerative medicine therapeutic approaches and study a wide variety of diseases associated with chromosomal abnormalities, such as metabolic syndrome and type II diabetes, both of which are increasing in Saudi Arabia.
“We’ve created the first inducible pluripotent stem cell lines,” says Ph.D. student Maryam Alowaysi. “These have great potential because they can be differentiated into all known possible cells in the body. This means we can study the onset of disease and the genes associated with it.”
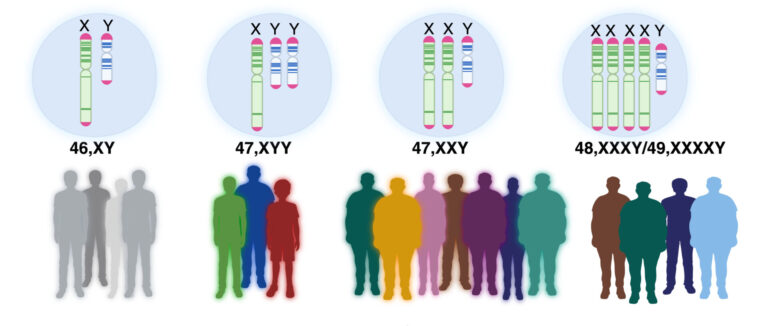
Klinefelter syndrome occurs when a male is born with one or more extra X chromosomes due to a random genetic error that happens during conception.
Klinefelter syndrome, also known as 47-XXY, occurs when a male is born with one or more extra X chromosomes due to a random genetic error that happens during conception. The most common sex chromosome disorder, it affects about one in 660 males worldwide.
People carrying more than two extra X chromosomes, such as those with 48-XXXY and 49-XXXXY karyotypes, display progressively worsening conditions, ranging from infertility and osteoporosis, to cancer and metabolic diseases, such as type II diabetes. Others may have mosaic Klinefelter syndrome, which means they only have an extra copy of the X chromosome in some cells.
While previous studies on Klinefelter syndrome have characterized the symptoms in detail, few have determined the genes associated with the condition. The wide variation in symptoms also makes accurate diagnoses difficult.
“Some patients may have type II diabetes, while others may have multiple sclerosis or infertility. They are common but highly variable characteristics, which is why diagnosis is very difficult,” says postdoc Elisabetta Fiacco. “If we can identify the pathway that affects specific genes that are in common, we can help shed light on the possible causes.”
The team used fibroblasts obtained from skin biopsies of patients with low- and high-grade Klinefelter syndrome. The researchers also derived cell lines from a patient with mosaic Klinefelter syndrome and a high-grade patient with a balanced translocation, a condition where sections from two chromosomes have swapped places.
They reprogrammed the fibroblast cells with nonmodified messenger RNAs to shift them from a somatic to a pluripotent state. “This means they have the potential to differentiate into virtually any germ layer of the body — endoderm, mesoderm and ectoderm,” says Alowaysi.
The team used various approaches to test the pluripotency of their generated cell lines, including immunofluorescence, reverse transcription PCR and teratoma assays — the gold standard validation technique for pluripotency.
The generated cell lines expressed the markers OCT4, NANOG and SOX2 at both the mRNA and protein level, confirming that the cells had been successfully reprogrammed to be pluripotent.
When the researchers injected the cells into mice, they formed tumors that expressed protein markers S100A1, Desmin and Cytokeratin, demonstrating that the cells are able to differentiate into all three germ layers.
“These cells are differentiated in a dish into virtually any cell type of the human body, which means we can detect the genes involved in the disease and compare them with healthy control cells,” says Fiacco. “With this, we can see that the genes are dysregulated or doing something that is not in the normal function of those genes.”
Now that the researchers have generated the Klinefelter syndrome cell lines, the next step is to analyze the transcriptomic profile to uncover the dysregulated genes in the cohort and their role in the molecular pathology of the condition.
“This work is partially supported by the KAUST Smart Health Initiative that aims, among its primary goals, to generate large cohorts of Saudi and non-Saudi patient-derived induced pluripotent stem cells,” says Antonio Adamo, assistant professor and principal investigator at KAUST’s Laboratory of Stem Cells and Diseases.
“Under this initiative, our laboratory is helping to establish a KAUST biobank that can provide invaluable cellular tools and models to support Saudi researchers within the Kingdom.”
References
- Alowaysi, M., Fiacco, E., Astro, V. & Adamo, A. Establishment of iPSC lines from a high-grade Klinefelter Syndrome patient (49-XXXXY) and two genetically matched healthy relatives (KAUSTi003-A, KAUSTi004-A, KAUSTi004-B, KAUSTi005-A, KAUSTi005-B, KAUSTi005-C). Stem Cell Research 49, 102098 (2020).| article
- Alowaysi, M., Fiacco, E., Astro, V. & Adamo, A. Establishment of an iPSC cohort from three unrelated 47-XXY Klinefelter Syndrome patients (KAUSTi007-A, KAUSTi007-B, KAUSTi009-A, KAUSTi009-B, KAUSTi010-A, KAUSTi010-B). Stem Cell Research 49, 102098 (2020).| article
- Fiacco, E., Alowaysi, M., Astro, V. & Adamo, A. Derivation of two naturally isogenic iPSC lines (KAUSTi006-A and KAUSTi006-B) from a mosaic Klinefelter Syndrome patient (47-XXY/46-XY) Stem Cell Research 49, 102098 (2020).| article
- Alowaysi, M., Fiacco, E., Astro, V. & Adamo, A. Generation of two iPSC lines (KAUSTi001-A, KAUSTi002-A) from a rare high-grade Klinefelter Syndrome patient (49-XXXXY) carrying a balanced translocation t(4,11) (q35,q23). Stem Cell Research 49, 102098 (2020).| article
You might also like

Bioscience
Robust workflow built for chemical genomic screening
Bioscience
Cell atlas offers clues to how childhood leukemia takes hold
Bioscience
Hidden flexibility in plant communication revealed
Bioscience
Harnessing the unintended epigenetic side effects of genome editing
Bioscience
Mica enables simpler, sharper, and deeper single-particle tracking

Bioengineering
Cancer’s hidden sugar code opens diagnostic opportunities
Bioscience
AI speeds up human embryo model research
Bioscience




