Plant Science
Cleaving through the heart of viral infections
A Cas protein that cleaves viral RNA has been harnessed in a CRISPR/Cas system to destroy prolific viral infections in crop plants.

A novel CRISPR/Cas system that can efficiently attack and destroy several prolific plant viruses has been developed by KAUST using tobacco plants and is now being extended to rice and other crops.
This research aims to reduce the impact of plant viral infections, which cause losses of 10-15 percent of the global crop yield every year.
CRISPR is a natural system found in bacteria that confers adaptive immunity by copying and “storing” elements of viral DNA, which remind the organism of that particular virus and ready it for a future attack. When the virus attacks again, the bacteria’s CRISPR system releases CRISPR RNAs, which are small molecules that guide CRISPR-associated (Cas) proteins to bind to the viral DNA and attack and destroy the virus.
Scientists have harnessed this system and developed CRISPR/Cas technology, most notably as a genome editing tool used to modify segments of DNA. Recently, scientists from KAUST developed CRISPR/Cas13, a promising method of quelling RNA-based viral attacks in plants.
“Most plant viral genomes are based on single-stranded RNA,” says Magdy Mahfouz, who supervised Ph.D. student Ahmed Mahas and postdoc Rashid Aman in this research project. “So we needed a Cas protein that would cleave RNA to destroy the viruses. Our first study used variant Cas13a, which attacked the turnip mosaic virus, but it was not efficient enough. We decided to search for an alternative Cas13 variant that was more robust.”
In their latest study, the team trialed nine Cas13 variants to assess specificity and efficiency against three RNA viruses in Nicotiana benthamiana plants.
“Our main challenge was to devise an efficient, easy-to-use assay system to help us screen all the variants quickly,” says Aman. “One variant in particular, CasRx, stood out as robust and highly virus-specific—meaning it only targeted viral RNA and did no damage to the plant itself.”
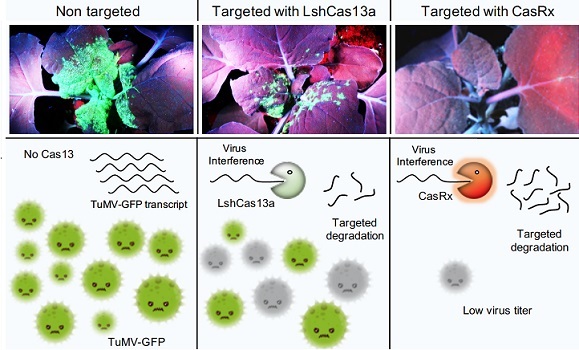
The variant CasRx (right) is robust and only targeted viral RNA, while doing no damage to the plant itself.
Reproduced under a Creative Commons license from reference 1 © 2020 Mahas et al.
“CasRx might be more efficient because it is one of the smallest Cas13 proteins found to date,” notes Mahas. “Unlike other Cas13 proteins, it doesn’t require binding to specific structures at the target RNA site, meaning it could access and degrade almost all the viral RNA sequences we tested,” he adds.
Furthermore, CasRx can target up to two viruses in the same plant at the same time. This would enable the simultaneous targeting of multiple viruses in mixed viral infections, like those found under natural conditions.
“We will harness CasRx to engineer different plant species against RNA- and DNA-based viruses,” says Mahas. “Our results also open up the potential for many other potential applications.”
References
-
Mahas, A., Aman, R. & Mahfouz, M. CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biology 20, 263 (2019).| article
You might also like

Plant Science
Coevolution of the microbiome with its host plant supports environmental adaptation
Bioscience
Hidden flexibility in plant communication revealed

Plant Science
Reference genomes for rice’s wild relatives may boost future crops
Bioscience
Digging into the world of plant-growth-promoting microbes
Environmental Science and Engineering
Hydrogen storage solution could lie in lakes

Bioscience
Unraveling modern bread wheat from the genes up

Bioscience
Why do some plants thrive in saline conditions?
Bioengineering




