Plant Science
Shaping up against pathogens
Plants can reprogram their genetic material to mount a defensive response against pathogens, which may have applications for agriculture.


Damage caused on tomato plants upon infection with Pseudomonas syringe bacteria.
© Chris Smart, NYSAES, Geneva NY
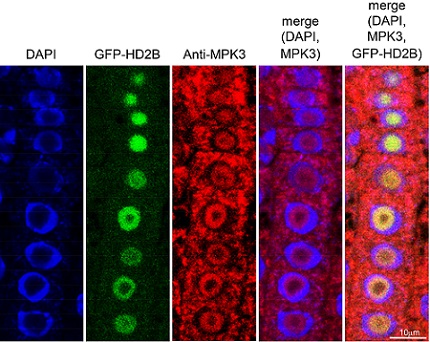
Immunofluorescence assays show the location of key components in the pathway to plant immunity: left, blue indicates cell nuclei; second left, HD2B within the nucleolus; center, MPK3 distributed both inside and outside the nuclei; second right and right, superimpositions of these images clearly show the differential location of HD2B and MPK3 within plant cells.
Reproduced with permission from ref 1.© Olga Samajova 2017
A missing link in the complex molecular pathway by which plants resist pathogens has been identified by an international team headed by Heribert Hirt from KAUST. This finding highlights a potential mechanism that could be harnessed to “vaccinate” crops against disease.
Plant defense is controlled by genes, made of DNA, which encode proteins. DNA is packaged into an aggregate called chromatin, the shape of which affects whether genes are available for protein building. Changes to the chromatin structure are epigenetic—potentially heritable without altering the DNA sequence itself—and have been associated with pathogen attack.
Pathogens produce small molecules called microbial-associated molecular patterns (MAMPs), which plants recognize via a bipartite receptor protein: part one senses MAMPs outside the plant cell and stimulates the second part inside the cell to act. The latter is a kinase, an enzyme that transfers phosphate groups onto target molecules (phosphorylation), and its action is the first in a sequence of MAMP-triggered phosphorylations between mitogen-activated protein kinases (MAPKs). Hirt, who is now a professor of plant science at KAUST, discovered these in 1997.
The link between MAPKs and epigenetic chromatin modification has remained elusive until this new finding by Hirt’s team, who included Assistant Professor Moussa Benhamed, serveral postdocs and a master’s student from the University’s Desert Agriculture Initiative.
Using the model species Arabidopsis thaliana, the researchers activated the MAPKs using a bacterial MAMP. Then, in a series of experiments dubbed “the phosphoproteomics pipeline,” they searched for phosphorylation events. Enticingly, they found that the final MAPK in the chain, MPK3, phosphorylates an enzyme, histone deacetylase (HD2B), which regulates DNA compaction into chromatin.
The team showed that, in mutant Arabidopsis lacking MPK3 or HD2B, many genes, including defense genes, increased in activity. This suggests that, normally, HD2B represses gene activity. Under pathogen attack, MPK3’s action on HD2B reverses this repression.
Using fluorescent-tagged HD2B, the team showed that MPK3 phosphorylation makes HD2B move from one cellular location to another, binding a different chromatin region. This pathway releases one set of genes and blocks a second set, fundamentally changing the cell’s molecular makeup. This epigenetic reprogramming is fast and reversible, enabling plants to respond rapidly to changing conditions and to retain a molecular memory facilitating a speedier response to future pathogens. According to Hirt, “epigenetic mechanisms are the mechanism of choice…the phenomenon occurs across all plants in response to all types of MAMPs and pathogens.”
Hirt believes kinase-triggered chromatin reprogramming is a widespread mechanism, and he is enthusiastic about possibilities for artificially stimulating this process. “Once we better understand the mechanism of inducing pathogen memory, we might be able to induce long-term resistance, similar to human vaccination,” he suggests. “We are entering a new era investigating the role of epigenetics in plant-stress memory.”
References
- Latrasse, D., Jégu, T., Huchen, L., de Zelicourt, A., Raynaud, C. … & Hirt, H. MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biology 18, 131 (2017).| article
You might also like

Plant Science
Coevolution of the microbiome with its host plant supports environmental adaptation

Bioscience
Hidden flexibility in plant communication revealed

Plant Science
Reference genomes for rice’s wild relatives may boost future crops
Bioscience
Digging into the world of plant-growth-promoting microbes
Environmental Science and Engineering
Hydrogen storage solution could lie in lakes

Bioscience
Unraveling modern bread wheat from the genes up

Bioscience
Why do some plants thrive in saline conditions?
Bioengineering



