Chemical Engineering
MOF metallic mastery
The tightly defined ratios of metals in metallic organic frameworks makes them ideal starting materials for novel catalyst creation.
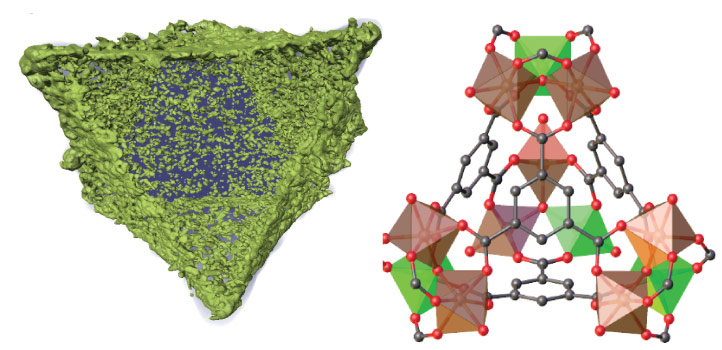
KAUST researchers develop a novel approach to catalyst design using metal organic frameworks. © 2021 KAUST. /en/article/1126/mof-metallic-mastery
KAUST researchers develop a novel approach to catalyst design using metal organic frameworks. © 2021 KAUST. /en/article/1126/mof-metallic-mastery
The tightly defined ratios of metals in MOFs makes them ideal starting materials for novel catalyst creation.
Heating bimetallic metal organic frameworks (MOFs) until their porous structure collapses into nanoparticles can be a highly effective way to make catalysts. This novel approach to catalyst design has now been used by KAUST and Spanish researchers to make a robust catalyst that converts carbon dioxide (CO2) into carbon monoxide (CO) gas with unprecedented selectivity.
The benefit of this method pioneered at KAUST is that it can generate mixed metal catalytic nanoparticles that have proven challenging or impossible to make by conventional means.
Capturing CO2 emissions and catalytically converting the greenhouse gas into CO, a valuable chemical feedstock, is one option for reducing greenhouse gases associated with climate change. Precious metals can catalyze this reaction, but they are costly and supplies are limited, says Samy Ould-Chikh, a research engineer in KAUST.
“Iron oxide catalysts are an inexpensive alternative,” Ould-Chikh says. “However, in the presence of CO, the iron is carburized forming iron carbide, which leads to by-product formation and catalyst deactivation.”
Adding titanium to the catalyst particles could stabilize iron oxide against carburization. Chemical incompatibilities between iron and titanium precursors, however, had made it impossible to synthesize nanoparticles incorporating a homogenous mixture of the two metals in the necessary ratio. To overcome this limitation, the team turned to MOFs, porous materials made from metal ions connected together by carbon-based linkers.
“The use of MOFs allows us to perfectly control the iron-titanium ratio on the parent MOF,” says research engineer Adrian Ramirez Galilea. Heating decomposes the organic part of the MOF, leaving the two metals behind, homogenously mixed in the desired ratio and in neat octahedral nanoparticles that mirror the structure of the parent MOF.
The nanoparticles converted CO2 to CO with 100 percent selectivity, with no sign of deactivation after several days of use. “Our initial calculations suggested that nanoparticles with such atomic ratios should be able to do the job; however, the results far exceeded our original expectations,” Gascon says.
As well as continuing to explore the properties and reactivity of the iron-titanium nanocatalyst, the team is examining other metal catalyst systems made from MOFs in the same way. “The use of MOFs opens the way to synthesize new catalysts that were not possible to make using conventional approaches,” Ramirez Galilea says.
“We are looking at different metal combinations for applications ranging from traditional thermal catalysis to photo and photothermal catalysis,” adds Jorge Gascon, who led the research. “This paper is just the tip of the iceberg.”
References
- Castells-Gil, J., Ould-Chikh, S., Ramirez, A., Ahmad, R., Prieto, G., Rodriguez Gómez, A., Garzon-Tovar, L., Telalovic, S., Liu, L., Genovese, A., Padial, N.M., Aguilar-Tapia, A., Bordet, P., Cavallo, L., Martí-Gastaldo, C. & Gascon, J. Unlocking mixed oxides with unprecedented stoichiometries from heterometallic metal organic frameworks for the catalytic hydrogenation of CO2. Chem Catalysis 1, 364-382 (2021).| article
You might also like

Chemical Engineering
Urban air pollution goes up in smoke
Chemical Engineering
Rethinking machine learning for frontier science

Chemical Engineering
Magnetic nanoparticles capture microplastics from water

Chemical Engineering
Biogas upgrading goes with a swing

Chemical Engineering
Stronger, lighter, cheaper: a new route to carbon fiber production

Chemical Engineering
Unveiling the role of biomass-burning aerosols in atmospheric reactions

Chemical Engineering
Slashing industrial emissions using a hybrid model approach
Chemical Engineering




