Electrical Engineering
Fixing a greenhouse gas for useful chemicals
A catalyst that helps develop a new route to break up carbon dioxide uses a renewable source of clean energy.

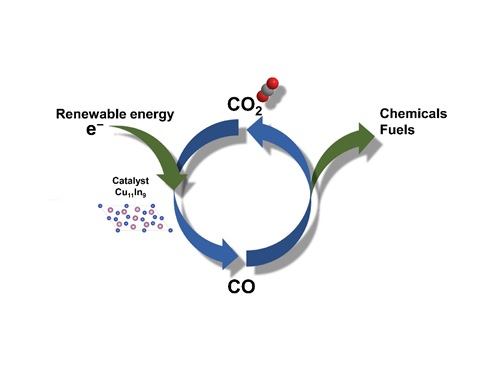
A catalyst made from an alloy of copper and indium increases the efficiency and selectivity of the process of converting carbon dioxide into carbon monoxide.
© 2015 KAUST
An alloy of copper and indium that helps the electrochemical conversion of carbon dioxide into carbon monoxide has been developed by a research team at KAUST1.
Clever solutions to technological solutions can often be found in nature. So when scientists and engineers first looked for ways to generate clean and renewable energy, they considered the evolution of plants which, through photosynthesis, harness the power of sunlight to convert carbon dioxide absorbed from the atmosphere into chemical energy in the form of sugars.
Researchers are aiming to replicate the process in artificial light harvesting devices. Crucial to the development of efficient artificial photosynthesis is a catalyst to increase the rate of the carbon dioxide conversion. “A catalyst in any process needs to to be efficient, selective, robust, and ubiquitous,” says research leader Kazuhiro Takanabe from KAUST’s Catalysis Center and the Advanced Nanofabrication Imaging and Characterization Core Lab.
One common method for converting carbon dioxide is to use electricity from renewable resources to generate other molecules including carbon monoxide, methane, formic acid and a host of other hydrocarbons. The proportions of these products depend heavily on the type of metals used. But a plethora of chemicals is not ideal: each needs to be treated differently to retrieve the energy.
Researchers needed to develop a catalyst with the requisite traits, while being selective in terms of the chemical products it helps to produce.
Takanabe and his colleagues fabricated a catalyst of an alloy of copper and indium that selectively produces carbon monoxide — a utilitarian product because it can be easily converted to other hydrocarbons as required.
Another advantage is that the catalyst does not require expensive materials such as silver and gold, reducing its cost relative to other catalysts that have been tried. To create the catalyst, the scientists reduced copper oxide in an indium sulfate solution, then tested it in an electrochemical containing aqueous potassium bicarbonate saturated with carbon dioxide.
They showed that the electrochemical process with their catalyst was highly selective producing 90 percent carbon monoxide, with hydrogen as the second most common byproduct.
Importantly, the process was also stable over time. “Ours is one of the best performing that satisfies all these requirements,” says Takanabe. “There is still margin for efficiency improvement and we will continue the search with other combinations of metals.”
References
-
Rasul, S., Anjum, D. H., Jedidi, A., Minenkov, Y., Cavallo, L. & Takanabe, K. A Highly selective copper–indium bimetallic electrocatalyst for the electrochemical reduction of aqueous CO2 to CO. Angewandte Chemie International Edition 54, 2146 –2150 (2015).| article
You might also like
Bioengineering
Self-aware biosensors boost digital health monitoring

Bioengineering
Smart patch detects allergies before symptoms strike

Computer Science
Green quantum computing takes to the skies
Electrical Engineering
Micro-LEDs boost random number generation

Bioengineering
Sensing stress to keep plants safe
Computer Science
Sweat-sniffing sensor could make workouts smarter

Electrical Engineering
New tech detects dehydration by touching a screen

Electrical Engineering



